- Single gene testing with Igenomix includes the possibility to individually analyse single genes that are that are associated with a specific clinical phenotype using different techniques including sanger sequencing. Our test menu represents all the clinically relevant genes that have been selected based on curated gene reviews, OMIM, variant databases (HGMD and ClinVar), most recent literature, and customer requests.Testing for single gene disorders can be done using several different technologies depending on the gene and disease in question.
SANG – Sanger/MiniSeq Sequencing

Single gene testing is recommended for patients with:
- Distinctive clinical features
- Family history of a specific disorder
- Single gene disorders
- Family testing confirmation
The applications of Sanger Sequencing include:
- Confirming single nucleotide variants that have been identified in other genetic tests.
- Carrier testing of known familial variants.
- When other types of genetic testing methods are not appropriate.
There are two possible results that can be obtained from sanger sequencing:
- A positive result indicating that the single nucleotide variant has been identified either in the homozygous or heterozygous state.
- A negative result indicating that the single nucleotide variant has not been identified.
Sample Requirements
For genetic testing through next generation sequencing, the following sample types are accepted. A thorough labelling of the tube with unique identifying information is suggested, incorrect labelling can lead to rejection of the sample. The minimum required information to identify and accept a sample is – Patient’s full name, Date of birth, Gender and Medical Record Number.
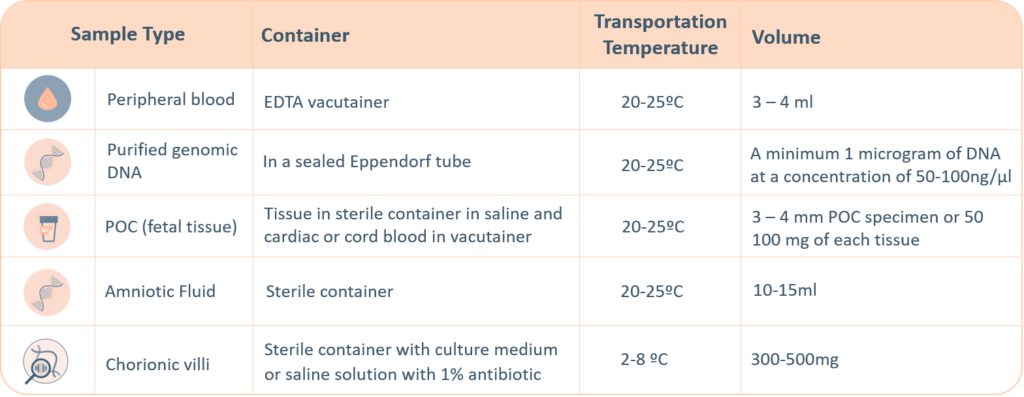
- Maternal blood sample must be sent with all products of conception, CVS and Amnio samples
- Precedence will be given to all prenatal samples
The ‘informed consent’ form and the ‘test requisition from’ (included within the provided kit) must be properly filled-in and signed by the patient and sent with the samples inside the shipping box or by e-mail to the laboratory. Igenomix will send you all the documents needed for the pick-up and transportation of the appropriate kit to our laboratory.
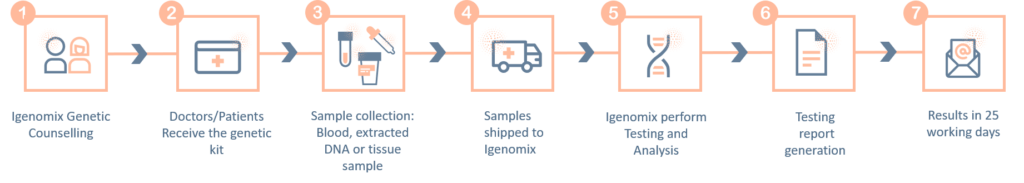
Methodology
Limitations
-
- Sanger sequencing is a gene-specific test and can only analyse a small subset of genes.
- Sanger sequencing is able to identify mosaic mutations present in as low as 20% of the cells, but Sanger sequencing is not precisely quantifiable and additional testing strategies must be used for quantification.
- Sanger sequencing can not readily identify partial or whole gene deletions.
- Medium-sized deletions larger than the PCR amplicons are not detected because they cannot be amplified appropriately.
Why Choose IGENOMIX?
High-Quality interpretation
Genetic counselling E-learning platform
Easy & Fast Online tests management
Local Labs over the Middle East
All Technical capabilities In-House
100% In-House sequencing and Bio IT
CAP & ISO accredited lab





