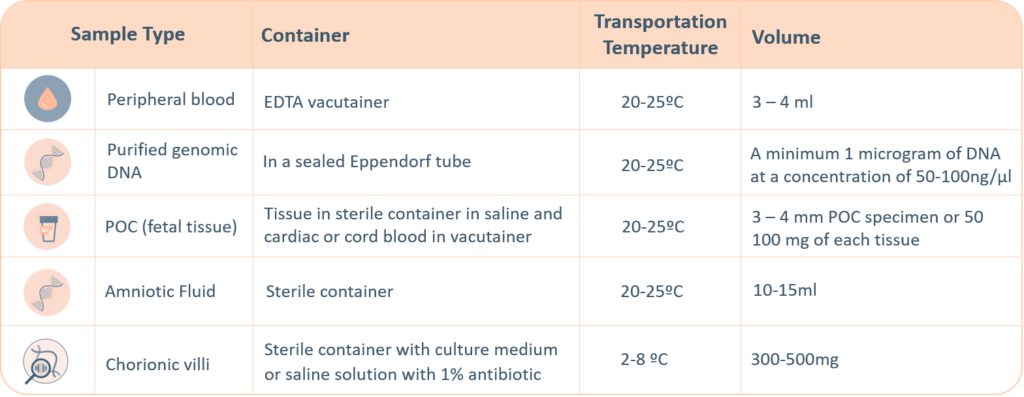
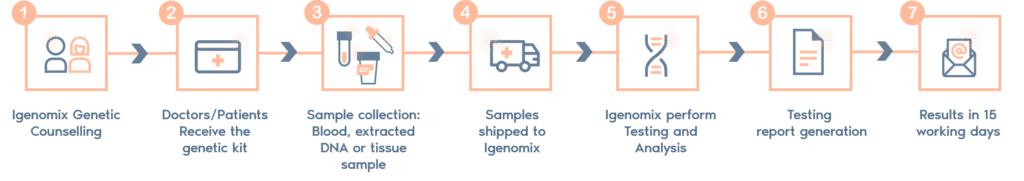
-
Multiplex Ligation dependent Probe Amplification (MLPA) is a multiplex PCR method used to detect abnormal copy numbers in specific and previously known regions of the genome that are associated with a specific genetic condition.
-
MS-MLPA is a technologic MLPA adaptation for detection of DNA methylation changes associated to genetic disorders.
-
Is the most reliable and cost-effective method of detecting known deletion, duplications, and specific copy number variations (CNVs). The probes or probe kits used have been selected and validated to reduce the likelihood of false positive or negative results.
MLPA – Multiplex ligation-dependent probe amplification
For CNVs in a gene or genomic region