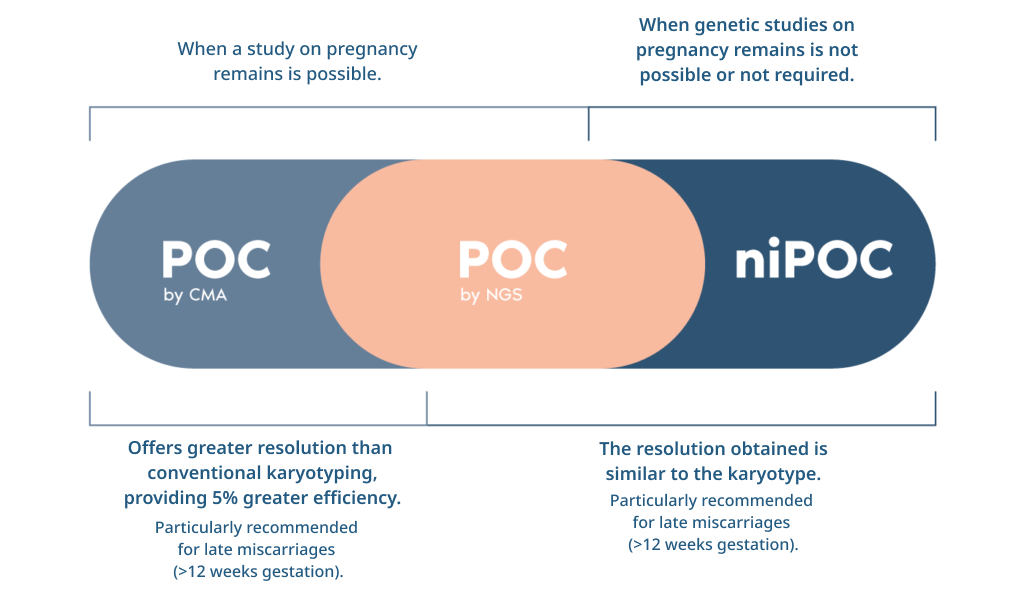
What is the POC test on pregnancy remains?
The family of POC tests analyse whether the miscarriage could have been caused by a
chromosomal abnormality.
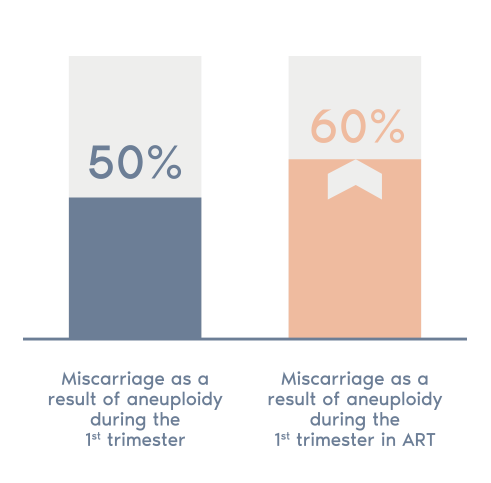
- 50% of miscarriages in the first trimester present abnormalities in the number of
chromosomes (aneuploidies). - This figure rises to 60% in cases of assisted reproductive treatment and further increases with age.
- The POC test analyses the 24 chromosomes and is able to identify alterations to the number of chromosomes (aneuploidies) as well as losses and gains of chromosome fragments.
- Complementary analysis of STR (Short Tandem Repeats) microsatellite markers
in the patient’s blood enables us to identify ploidy alterations (haploid, triploid,
tetraploid) and determine the origin of the tissue under study (foetal or
maternal).
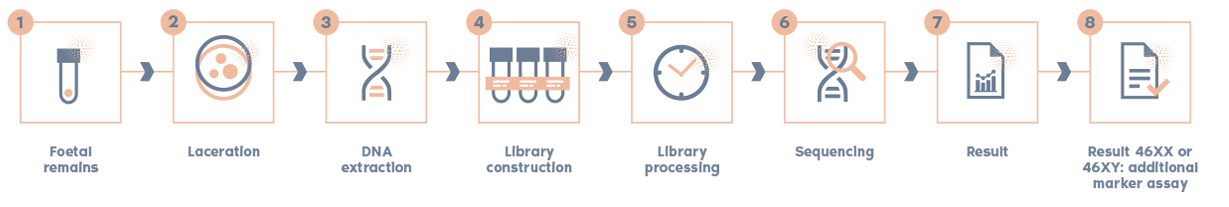
How is the POC test on pregnancy remains performed?
There are different methods depending on the test carried out:
ni-POC Methodology
The blood sample is obtained with the same procedure as in a routine analysis
POC Methodology
Main steps of the POC Analysis